
The trial will continue to analyze DFS in patients whose tumors express high levels of PD-L1 (TPS ≥50%) and evaluate overall survival, a key secondary endpoint. The Phase 3 KEYNOTE-091 trial investigating anti-PD-1 immunotherapy treatment with Keytruda (pembrolizumab), improved disease-free survival (DFS) when used as adjuvant treatment of patients with stage IB-IIIA non-small cell lung cancer (NSCLC) following surgical resection regardless of PD-L1 expression. Keytruda also crosses the blood - brain barrier and ~ 30% of cancers in the brain of individuals that express PD-L1 will respond to treatment. This was the second clinical study demonstrating that the combination of a checkpoint inhibitor combined with chemotherapy improves treatment outcomes for NSCLC compared to treatment with chemotherapy alone. The 36-month overall survival is 43.7% for Keytruda compared to 24.9% for chemotherapy. The average overall survival duration among Keytruda treated patients is now 26.3 months compared to 14.2 months for those treated with chemotherapy.

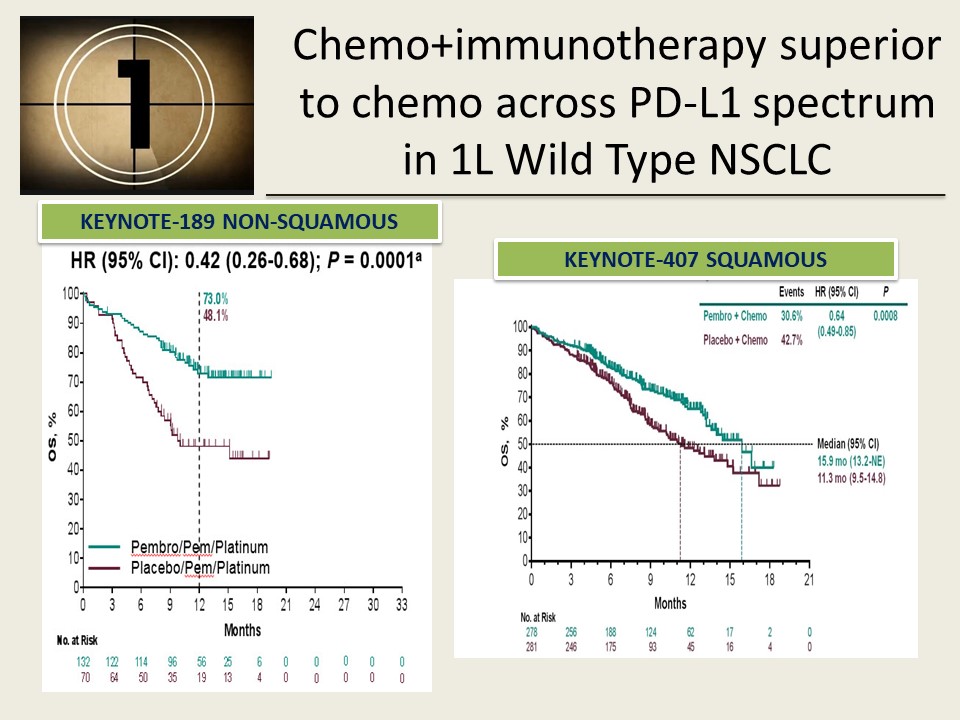
Based on these results the trial was stopped, and patients receiving chemotherapy in KEYNOTE-024 were offered the opportunity to receive Keytruda.įollow up at three years from initiation of treatment was released in September 2019 at The International Association for the Study of Lung Cancer (IASLC) annual meeting. It was initially reported that Keytruda was superior compared to chemotherapy for delaying the time to cancer progression-and improving overall survival. Patients treated with platinum based chemotherapy had the option of crossing over to Keytruda upon disease progression. The study enrolled 305 patients to receive Keytruda or platinum-based chemotherapy: paclitaxel+carboplatin, pemetrexed+carboplatin, pemetrexed+cisplatin, gemcitabine+carboplatin, or gemcitabine+cisplatin. The KEYNOTE-024 a clinical trial directly compared Keytruda to standard platinum-based chemotherapy in the treatment of patients with advanced NSCLC whose tumors expressed high levels of PD-L1. Keytruda Superior to Chemotherapy as Initial Treatment of Advanced Disease

A diagnostic test to measure the level of PD-L1 is available. By blocking the binding of the PD-L1 ligand these drugs restore an immune cells’ ability to recognize and fight the colon cancer cells. A checkpoint inhibitor can block the PD-1 and PD-L1 pathway and enhance the ability of the immune system to fight cancer. PD-1 and PD-L1 are proteins that inhibit certain types of immune responses, allowing cancer cells to evade detection and attack by certain immune cells in the body. Keytruda is a precision cancer medicine that belongs to a class of medicines called “checkpoint inhibitors.” Checkpoint inhibitors are a novel precision cancer immunotherapy that helps to restore the body’s immune system in fighting cancer by releasing checkpoints that cancer uses to shut down the immune system. Results from studies evaluating immune-modulatory approaches using anti-PD-1 and anti-PD-L1 antibodies have demonstrated promising results and are advancing the standard of care for lung cancer. As a result, patients with lung cancer now typically receive molecular testing that guides their physicians in determining which therapies are more likely to boost the chances of survival while limiting the potential for adverse effects. Tailored treatments have emerged to match a person’s genetic makeup or a tumor’s genetic profile. Precision cancer medicines continues to impact the lives of lung cancer patients with research into genomics and genetics leading to unprecedented progress in improving outcomes. Although progress has been made in recent years, the majority of patients with advanced stage lung cancer still die from their disease.

In the United States, NSCLC accounts for 75–80% of all lung cancers. Lung cancer remains the leading cause of cancer-related deaths worldwide.


 0 kommentar(er)
0 kommentar(er)
